42 what is an open label study
Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] Understanding Clinical Trial Terminology: What is a Long-Term Extension ... An OLE study is a clinical trial that typically enrolls participants of a previous clinical trial and is designed to gather the long-term safety and tolerability data on a potential new medicine beyond the time period of the main study.
Cassava Sciences Reports Second Quarter Financial ... - Yahoo! Aug 03, 2022 · Open-label Study – Results of an Interim Analysis on the First 100 Patients Who Have Completed at Least 12 Months of Open-label Treatment with Simufilam Follow: Drug Appears Safe and Well Tolerated.

What is an open label study
Open label extension studies: research or marketing? - PMC Open label extension studies are a common adjunct to double blind randomised controlled trials of new drugs The aim of open label extension studies often seems to be to enable continued use of a new drug for marketing or compassionate purposes rather than to increase knowledge Safety and Immunogenicity Study of 2019-nCoV 25.02.2020 · This is a phase I, open-label, dose-ranging clinical trial in males and non-pregnant females, starting at 18 years of age, inclusive, who are in good health and meet all eligibility criteria. This clinical trial is designed to assess the safety, reactogenicity and immunogenicity of mRNA-1273 manufactured by ModernaTX, Inc. mRNA-1273 is a novel lipid nanoparticle (LNP) … Study of Iadademstat and Gilteritinib in Patients With R/R AML With FMS ... Escalation/extension open label study: Masking: None (Open Label) Primary Purpose: Treatment: Official Title: An Escalation/Expansion, Open Label, Multicenter Study of Iadademstat and Gilteritinib in Patients With Relapsed or Refractory Acute Myeloid Leukemia (R/R AML) With FMS-like Tyrosine Kinase Mutation (FLT3 Mut+): The FRIDA Study ...
What is an open label study. A Multicenter, Open-Label, Phase 3 Trial to Compare the Efficacy … 04.01.2013 · E7080-G000-304 is a multicenter, randomized, open-label, noninferiority Phase 3 study to compare the efficacy and safety of lenvatinib versus sorafenib as a first-line systemic treatment in participants with unresectable Hepatocellular Carcinoma (HCC). Condition or disease Intervention/treatment Phase ; Hepatocellular Carcinoma (HCC) Drug: Lenvatinib Drug: … Meaning of "Open Label Pilot study"..?? - SAS Open label is a term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving. Cassava Sciences Reports Second Quarter Financial Results for 03.08.2022 · Open-label Study – Results of an Interim Analysis on the First 100 Patients Who Have Completed at Least 12 Months of Open-label Treatment with Simufilam Follow: Drug Appears Safe and Well Tolerated. Open-Label Extension Studies | SpringerLink As the name implies, an open-label extension study is an 'appendage' to a randomised controlled clinical trial, usually of an unregistered medicine or intervention. Often the drug is being studied under an investigational new drug (IND) licence or equivalent legislation. The open-label extension study is identified formally as a study.
End of Trial and Open-Label Extension (OLE) Frequently Asked Questions ... Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time. Open-Label Trial - an overview | ScienceDirect Topics Open Label Studies. In open label trials, both the study participant (and caregiver where applicable) and the investigator are aware of the treatment provided to study participants. A common use for this design is in an extension trial, which immediately follows a randomized controlled trial, and is used for gathering safety data in the same ... A phase 3, open-label, randomized study of asciminib, a STAMP ... - PubMed 25.11.2021 · Fewer grade ≥3 adverse events (50.6% vs 60.5%) and adverse events leading to treatment discontinuation (5.8% vs 21.1%) occurred with asciminib than with bosutinib. The study showed a superior efficacy of asciminib compared with that of bosutinib, together with a favorable safety profile. These results support the use of asciminib as a new ... Open-label extension studies: do they provide meaningful ... - PubMed Consumers, institutions where these studies are conducted and research ethics committees need to be convinced of the motives, as well as the quality, of the open-label extension study and its execution before supporting such studies. Open-label extension studies do have a legitimate but limited place in the clinical development of new medicines.
PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology factors. In the case of open-label extension studies, we feel that analysis of efficacy ought to regard treatment allocation as a possible explanatory variable (in addition to others, such as baseline status and time). The study by Mease and colleagues 1 reports the results of a 48-week open-label extension of a 24-week randomized Pemigatinib for previously treated, locally advanced or ... - PubMed Background: Fibroblast growth factor receptor (FGFR) 2 gene alterations are involved in the pathogenesis of cholangiocarcinoma. Pemigatinib is a selective, potent, oral inhibitor of FGFR1, 2, and 3. This study evaluated the safety and antitumour activity of pemigatinib in patients with previously treated, locally advanced or metastatic cholangiocarcinoma with and without FGFR2 … What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded." If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open." After recruitment to the trial, the participants were allocated to treatment using block randomisation. (PDF) What is an open label trial? - ResearchGate What is an open label trial? Authors: Philip M. Sedgwick St George's, University of London Abstract Researchers assessed the effectiveness of prazosin combined with scorpion antivenom in assisting...
NOPARK Open Label Extension Study - Full Text View - ClinicalTrials.gov The NOPARK open label extension study is an open label study, where subjects enrolled in the NOPARK study (ClinicalTrials.gov: NCT03816020), upon completion of the study, will be offered the NR study drug for continuous use, until the NOPARK study is completed and the primary end-point assessed (31/12/2025). The dose of NR is 12000mg NR daily ...
Open label study | definition of open label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc.
A Randomized Controlled Open Label Crossover Trial to Study Vaginal ... Lactobacilli administration has been suggested for the treatment and prevention of bacterial vaginosis, which increases the risk for preterm birth. We aimed to evaluate the vaginal colonization of lactobacilli orally administered to pregnant women at risk for preterm birth. We performed a randomized and controlled crossover study between January 2016 and May 2017. Forty pregnant women at high ...
Open-Label Trial - an overview | ScienceDirect Topics Open-label extension studies: In these studies, which often follow a double-blind randomized placebo-controlled trial, subjects have the option of remaining on the study intervention in an open-label fashion (i.e., they know that they are on the study intervention) for an extended period of time (e.g., several years). They may be informed of ...
What is an open label extension study? • NCK Pharma An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered.
Effects of open-label placebos in clinical trials: a ... - Nature Open-label placebos (OLPs) are placebos without deception in the sense that patients know that they are receiving a placebo. The objective of our study is to systematically review and analyze the ...
What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials can be used to gather additional safety and efficacious data on drugs on the market to increase the confidence of clinicians, patients, and clinical bodies. They can play a key...
Reducing bias in open-label trials where blinded outcome assessment is ... Many trial designs do not permit blinding, and are therefore designed as open-label, with patients, clinicians, and other study investigators aware of treatment allocation. Research has suggested that these trials should use blinded outcome assessment to avoid bias in estimated treatment effects [6-10]. Blinded outcome assessment is often ...
Open Label Extension Study Definition | Law Insider Examples of Open Label Extension Study in a sentence. Rigel shall conduct such Open Label Extension Study in a good scientific manner, in compliance in all material respects with all Applicable Laws and all applicable portions of the Transition Plan.. AZ shall be solely responsible for the development of the Products in the Field in the Territory, and shall assume responsibility to fund the On ...
Open-label study | definition of open-label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc.
National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine.
Open-label Definition & Meaning - Merriam-Webster open-label: [adjective] being or relating to a clinical trial in which the treatment given to each subject is not concealed from either the researchers or the subject — compare double-blind, single-blind.
Medical Definition of Open-label - MedicineNet Open-label: A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving. CONTINUE SCROLLING OR CLICK HERE SLIDESHOW
Open-Label Trial | NIH - HIV.gov Open-Label Trial A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants.
Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.
Open-label study - definition of Open-label study by The Free Dictionary Open-label study synonyms, Open-label study pronunciation, Open-label study translation, English dictionary definition of Open-label study. n. pl. stud·ies 1. a. The effort to acquire knowledge, as by reading, observation, or research: The study of language has overturned many misconceptions.
Study of Iadademstat and Gilteritinib in Patients With R/R AML With FMS ... Escalation/extension open label study: Masking: None (Open Label) Primary Purpose: Treatment: Official Title: An Escalation/Expansion, Open Label, Multicenter Study of Iadademstat and Gilteritinib in Patients With Relapsed or Refractory Acute Myeloid Leukemia (R/R AML) With FMS-like Tyrosine Kinase Mutation (FLT3 Mut+): The FRIDA Study ...
Safety and Immunogenicity Study of 2019-nCoV 25.02.2020 · This is a phase I, open-label, dose-ranging clinical trial in males and non-pregnant females, starting at 18 years of age, inclusive, who are in good health and meet all eligibility criteria. This clinical trial is designed to assess the safety, reactogenicity and immunogenicity of mRNA-1273 manufactured by ModernaTX, Inc. mRNA-1273 is a novel lipid nanoparticle (LNP) …
Open label extension studies: research or marketing? - PMC Open label extension studies are a common adjunct to double blind randomised controlled trials of new drugs The aim of open label extension studies often seems to be to enable continued use of a new drug for marketing or compassionate purposes rather than to increase knowledge





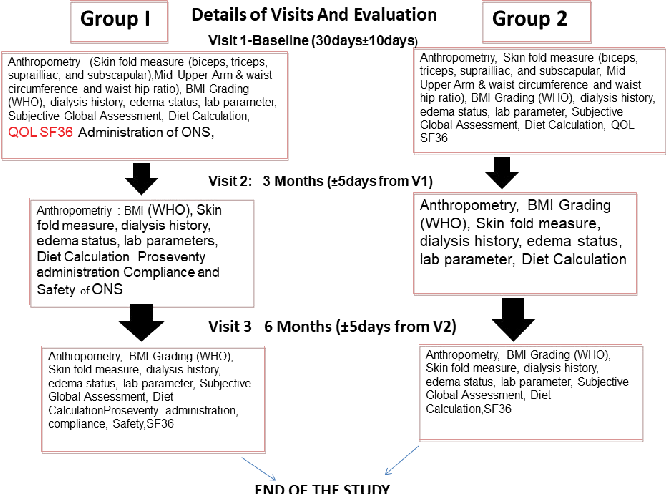


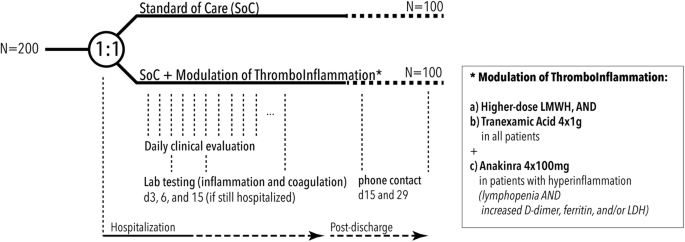








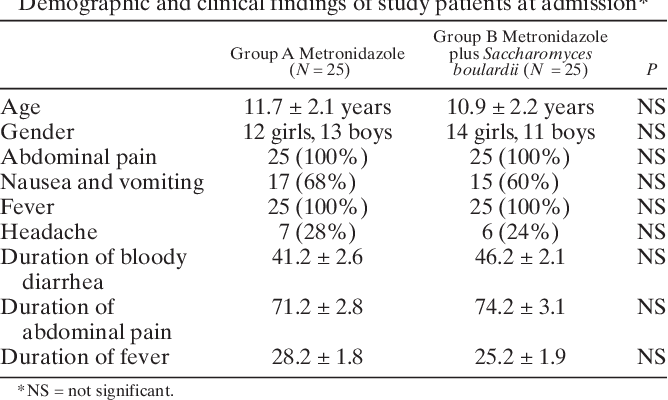
















![PDF] ZINC HYALURONATE IN THE TREATMENT OF DIABETIC FOOT ...](https://d3i71xaburhd42.cloudfront.net/fc5a851ceb2630c27c4ef0786beda8efd5be5fae/3-Table1-1.png)


Post a Comment for "42 what is an open label study"