44 open-label study disadvantages
What is an open label trial? | The BMJ Researchers assessed the effectiveness of prazosin combined with scorpion antivenom in assisting recovery from scorpion sting. An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Open-label extension studies: do they provide meaningful ... - PubMed Negative aspects of open-label extension studies revolve around their use as a marketing tool, as they build a market for the drug and generate pressure for subsidised access to the drug from consumers and their physicians.
Open-Label Extension Studies | SpringerLink The reputation of open-label extension study has been damaged by studies for which this has been the motivation. They have commonly been distinguished by rudimentary data collection and quality, revealing the true motive, namely market expansion.

Open-label study disadvantages
Open-Label Trial - an overview | ScienceDirect Topics Open Label Studies. In open label trials, both the study participant (and caregiver where applicable) and the investigator are aware of the treatment provided to study participants. A common use for this design is in an extension trial, which immediately follows a randomized controlled trial, and is used for gathering safety data in the same ... Pilot Studies: Common Uses and Misuses | NCCIH For most interventions proposed by NCCIH investigators, suspected safety concerns are quite minimal/rare and thus, unlikely to be picked up in a small pilot study. If any safety concerns are detected, group-specific rates with 95 percent confidence intervals should be reported for adverse events. End of Trial and Open-Label Extension (OLE) Frequently Asked Questions ... Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time.
Open-label study disadvantages. Understanding Clinical Trial Terminology: What is an Open Label ... Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. In some instances, patients who complete one clinical trial may be eligible to continue in an open-label extension study where all participants are eligible to receive active treatment for an ... Epidemiology and Clinical Research Design, Part 1: Study Types. It is difficult to ensure that the exposed and control groups have the same risk of outcome (other than the exposure). In addition to known baseline characteristics for which the exposed and control groups are matched, there may be unknown prognostic factors that are unevenly distributed in the 2 groups contributing to the outcome. PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology In open-label assessment studies, there is a sig-nificant risk of biased assessment. Analysis of all subjects who were randomized (intent to treat analysis) is another important technique, since subjects who drop out can differ in crucial ways from subjects who remain in the study. In open-label extension studies, only a proportion of the sub- Statistical controversies in clinical research: limitations of open ... Open-label studies overestimated the risk of vascular adverse events with AA by at least 50%. Meta-analyses assessing adverse drug events should therefore be restricted to DB randomized trials. Keywords antiangiogenics vascular adverse drug events open-label trial double-blind trial reporting biais meta-analysis Key Message
Effects of open-label placebos in clinical trials: a ... - Nature Eleven trials were eligible for meta-analysis. These trials assessed effects of OLPs on back pain, cancer-related fatigue, attention deficit hyperactivity disorder, allergic rhinitis, major... What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials are insufficient for providing data on these reactions. Open-label trials can increase the confidence about incidence rates, but as they are typically biased and uncontrolled,... Open-Label Trial - an overview | ScienceDirect Topics Open-label extension studies: In these studies, which often follow a double-blind randomized placebo-controlled trial, subjects have the option of remaining on the study intervention in an open-label fashion (i.e., they know that they are on the study intervention) for an extended period of time (e.g., several years). They may be informed of ... Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized.
Crossover trials: what are they and what are their advantages and ... One can say that study participants serve as their own control. This leads to another advantage which is less study participants are required compared to a standard parallel randomized controlled trial (RCT). Reduction of sample size is consistent with the principle in medical research to use resources wisely. Treatment Use of Investigational Drugs | FDA There are four requirements that must be met before a treatment IND can be issued: 1) the drug is intended to treat a serious or immediately life-threatening disease; 2) there is no satisfactory ... Statistical controversies in clinical research: limitations of open ... Open-label studies overestimated the risk of vascular adverse events with AA by at least 50%. Meta-analyses assessing adverse drug events should therefore be restricted to DB randomized trials. Keywords antiangiogenics vascular adverse drug events open-label trial double-blind trial reporting biais meta-analysis Introduction Reducing bias in open-label trials where blinded outcome assessment is ... Many trial designs do not permit blinding, and are therefore designed as open-label, with patients, clinicians, and other study investigators aware of treatment allocation. Research has suggested that these trials should use blinded outcome assessment to avoid bias in estimated treatment effects [ 6 - 10 ].
Guidance for Clinical Trial Sponsors - Food and Drug Administration studies (see 21 CFR 312.50 and 312.56 for drugs and biologics, and 21 CFR 812.40 and 21 CFR ... that some approaches have particular advantages or disadvantages. In this document, we
External and internal validity of open label or double-blind trials in ... In these trials, open-label or double-blind double-dummy designs are being used to evaluate the efficacy and safety in prevention and treatment of venous thromboembolism or stroke prevention in atrial fibrillation in several thousands of patients.
End of Trial and Open-Label Extension (OLE) Frequently Asked Questions ... Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time.
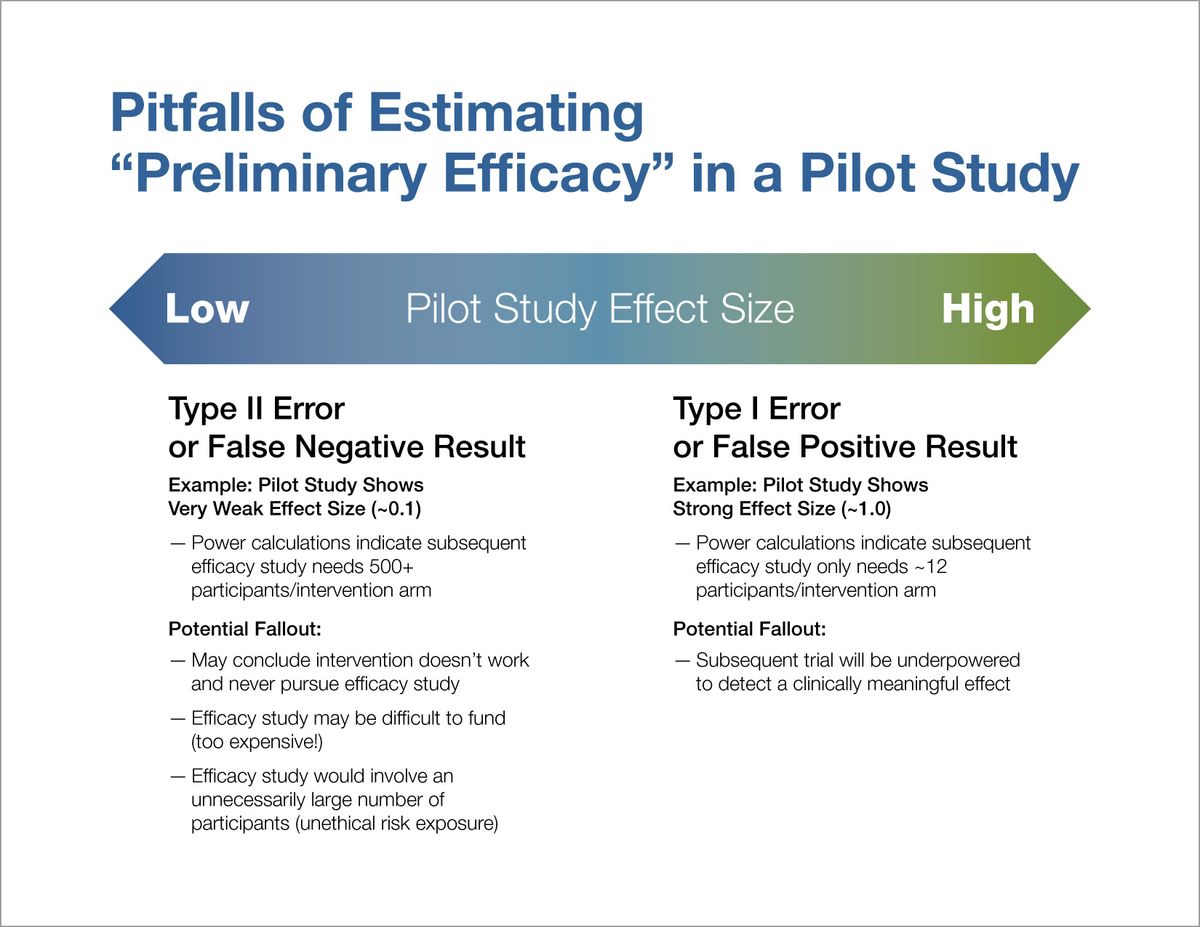
Pilot Studies: Common Uses and Misuses | NCCIH For most interventions proposed by NCCIH investigators, suspected safety concerns are quite minimal/rare and thus, unlikely to be picked up in a small pilot study. If any safety concerns are detected, group-specific rates with 95 percent confidence intervals should be reported for adverse events.
Open-Label Trial - an overview | ScienceDirect Topics Open Label Studies. In open label trials, both the study participant (and caregiver where applicable) and the investigator are aware of the treatment provided to study participants. A common use for this design is in an extension trial, which immediately follows a randomized controlled trial, and is used for gathering safety data in the same ...



![PDF] The Clinical Viewpoint: Definitions, Limitations of ...](https://d3i71xaburhd42.cloudfront.net/0d341492d445073e64d1ccb6985e4ad763a3d1f7/2-Table1-1.png)

























Post a Comment for "44 open-label study disadvantages"