39 what does exempt human specimen mean
Exempt Categories | Human Research Protection Program | Michigan State ... Exempt 4. Collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens if publicly available or information is recorded by investigator in a manner that subjects cannot be identified. Exempt 5. Federal demonstration projects. Exempt 6. Taste and food quality evaluation and consumer acceptance studies. PDF The ABCs of 104: Understanding Exemption Categories - HHS.gov • the study meets the regulatory definition for human subjects researchbut satisfies the conditions for one or more of the eight exempt categories described in the common rule • exempt studies are excused from the typical requirements of the common rule, such as, irb review according to the criteria at 46.111 and the informed consent requirements …
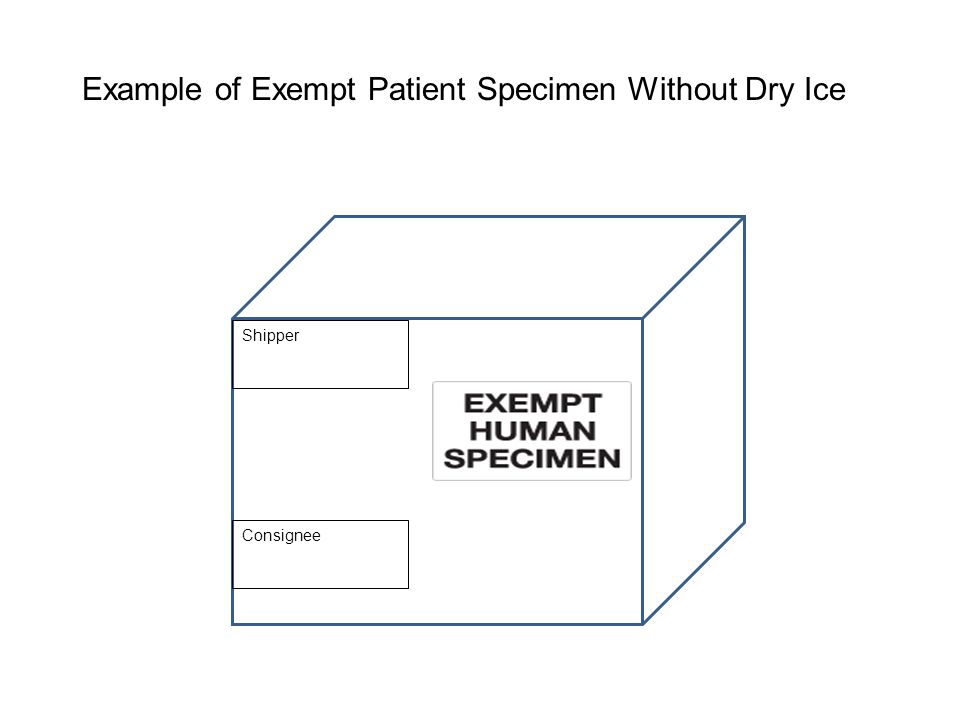
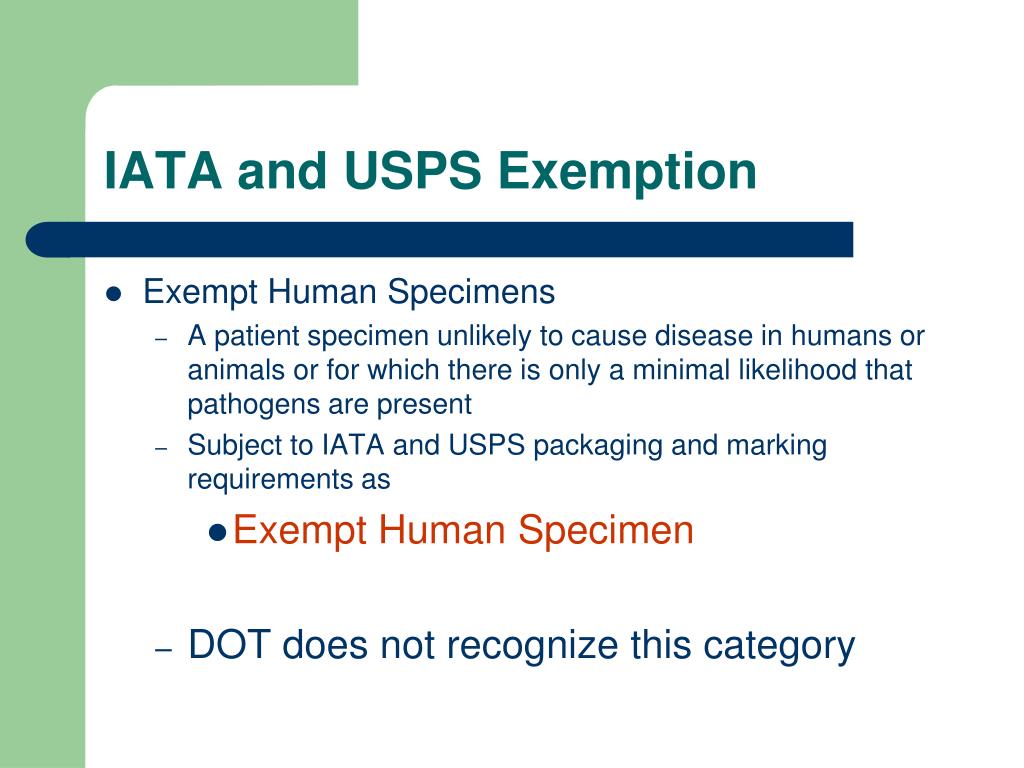
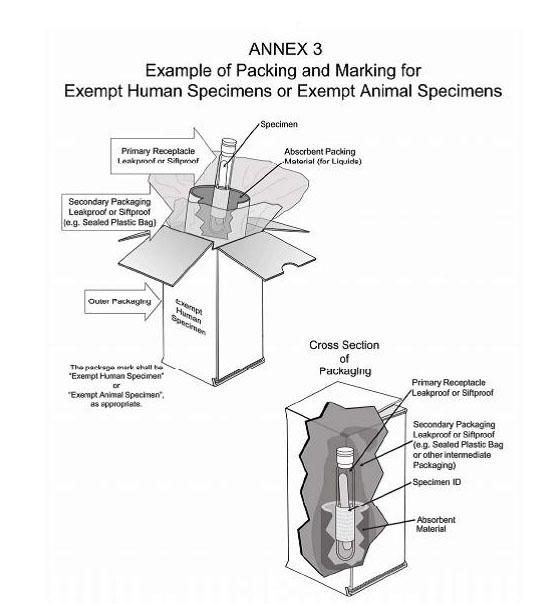
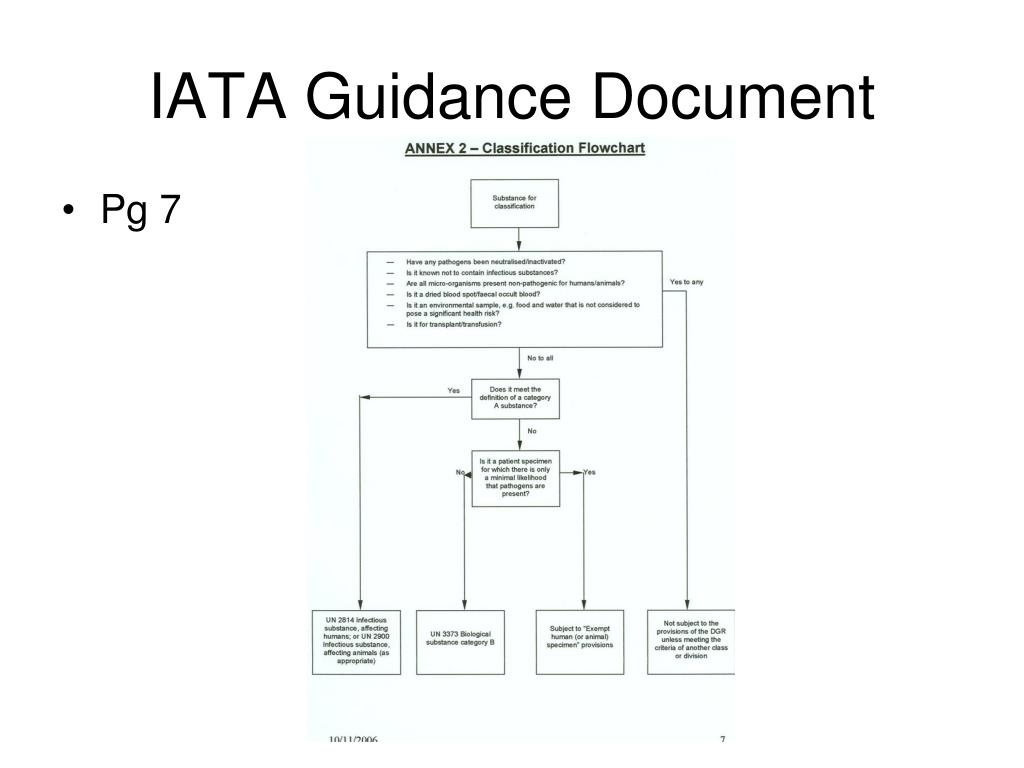
Exempt patient specimens - un3373.it EXEMPT HUMAN SPECIMEN or EXEMPT ANIMAL SPECIMEN. They are collected directly from humans or animals and there is minimal likelihood that pathogens are present. An element of professional judgment is required to determine if a substance is exempt under this paragraph. That judgment should be based on the known medical history, symptoms and individual circumstances of the source, human or animal, and endemic local conditions.
What does exempt human specimen mean
PDF QUICK GUIDE - Using Human Biological Specimens at UC Davis identifiers linked to the specimens. If the investigator does not record the identifiers or link them to the specimens, the research may be eligible for review under the exempt category. Specifically, exempt category #4 applies to research that involves the collection or study of existing* data, Waived Tests | CDC Waived tests include test systems cleared by the FDA for home use and those tests approved for waiver under the CLIA criteria. Although CLIA requires that waived tests must be simple and have a low risk for erroneous results, this does not mean that waived tests are completely error-proof. Errors can occur anywhere in the testing process ... Boxes - Sarstedt What do P650 "light" and "exempt human specimen" mean? This is an exemption under category B (infectious substances allocated UN3373), but only applies if there is a professional assessment (e.g. from a doctor) stating that the patient samples to be shipped have no or minimal likelihood of containing a pathogen.
What does exempt human specimen mean. Domestic Mail - USPS Exempt human or animal specimen means a human or animal sample (including, but not limited to, secreta, excreta, blood and its components, tissue and tissue fluids, and body parts) transported for routine testing not related to the diagnosis of an infectious disease. Typically, exempt human specimens are specimens for which there is a low ... PDF Proper Shipment of Patient Specimens and Infectious Substances Patient Specimens (minimal likelihood that pathogens are present) N/A N/A N/A See Checklist on Pg.6 Exempt Human Specimen or Exempt Animal Specimen N/A N/A N/A Non-infectious specimens (mammals, birds, amphibians, reptiles, fish, insect and other invertebrates) containing small quantities of flammable preservative Shipping Infectious Substances - Transport Canada In this case, you may ship the sample as "Exempt Human Specimen" if the medical professional has no reason to believe that the person has been in contact with an infectious substance. Examples of specimens that may be transported under this section include: Blood or urine specimens to monitor cholesterol levels, blood sugar levels or hormone ... Attachment B - Recommendations | HHS.gov the exemption is an acknowledgment that a subset of research activities that are already protected by hipaa—secondary research involving protected health information ("phi")—already afford human subjects rigorous regulatory protection of their privacy and that aside from privacy risks, these activities typically have lower overall human subjects …
Definitions | ORI - The Office of Research Integrity - HHS.gov The need for approval rests on three seemingly obvious but not always easy-to-interpret considerations: 1) whether the work qualifies as research, 2) whether it involves human subjects, and 3) whether it is exempt. All three considerations are discussed in the Common Rule and guide decision making about the use of human subjects in research. What does the term "exempt" actually mean in human subjects research ... Human subjects research that is classified as "exempt" means that the research qualifies as no risk or minimal risk to subjects and is exempt from most of the requirements of the Federal Policy for the Protection of Human Subjects, but is still considered research requiring an IRB review for an exemption determination. What Is A Human Specimen Definition - WhatisAny "Exempt human or animal specimen" means a human or animal sample (including, but not limited to, secreta, excreta, blood and its components, tissue and tissue fluids, and body parts) transported for routine testing not related to the diagnosis of an infectious disease. What does 3373 mean? How to Ship Clinical Samples | FedEx Liquid clinical samples marking requirements Include a marking on the package that properly identifies the shipment as "Exempt Human Specimen" or "Exempt Animal Specimen" as appropriate to comply with current IATA and ICAO regulations. If you prefer, package markings may be in the form of a label. Back To Top Ready to ship? Create a shipment online
Category B - UN3373.com -Human or animal specimens for which there is minimal likelihood that pathogens are present are not subject to these Regulations if the specimen is transported in a packaging which will prevent any leakage and which is marked with the words "Exempt human specimen" or "Exempt animal specimen", as appropriate. USPS Packaging Instruction 6H | Postal Explorer "Exempt human or animal specimen" means a human or animal sample (including, but not limited to, secreta, excreta, blood and its components, tissue and tissue fluids, and body parts) transported for routine testing not related to the diagnosis of an infectious disease. Exempt Research Definition | Office of Research Oversight/Regulatory ... Categories of exempt research are stipulated in Federal regulations at 45 CFR46.101(b)(1-6) as follows: (i.) Research conducted in established or commonly accepted educational settings, involving normal educational practices, such as(i) research on regular and special education instructional strategies,or (ii) research on the effectiveness of or the comparison among instructional techniques ... Carriage of Dangerous Goods Manual - Main Exemptions - HSE 7. For limited quantity exemptions, the general requirements for packaging (to be of good quality and suitable etc) apply, but the packaging does not have to be "UN approved.3.4.4 has a particular requirement for certain Class 8 (corrosive) goods. There are particular LQ marking requirements (see ADR 3.4.7 and 3.4.8.
What is Exempt | NYU Wagner What is Exempt. At the beginning of in the federal regulations, there is a list of categories of research that are exempt from the regulations. These include (but are not limited to): Research conducted in established or commonly accepted educational settings, involving normal educational practices, such as (i) research on regular and special education instructional strategies, or (ii) research on the effectiveness of or the comparison among instructional techniques, curricula, or classroom ...
Product - Sarstedt What do P650 "light" and "exempt human specimen" mean? This is an exemption under category B (infectious substances allocated UN3373), but only applies if there is a professional assessment (e.g. from a doctor) stating that the patient samples to be shipped have no or minimal likelihood of containing a pathogen.
Definition of Human Subjects Research | grants.nih.gov Definition of Human Subjects Research. According to 45 CFR 46 , a human subject is "a living individual about whom an investigator (whether professional or student) conducting research: Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or.
PDF 1 Meets the definition of human subjects research. Exempt studies involve human subjects research: research involving a living individual about whom data or biospecimens are obtained/used/studied/analyzed through interaction/intervention, or identifiable,
PDF Exempt Human Specimen / Exempt Animal Specimen Reference Guide (IATA 3 ... (11) A human or animal sample (including, but not limited to, secreta, excreta, blood and its components, tissue and tissue fluids, and body parts) being transported for routine testing not related to the diagnosis of an infectious disease, such as for drug/alcohol testing, cholesterol
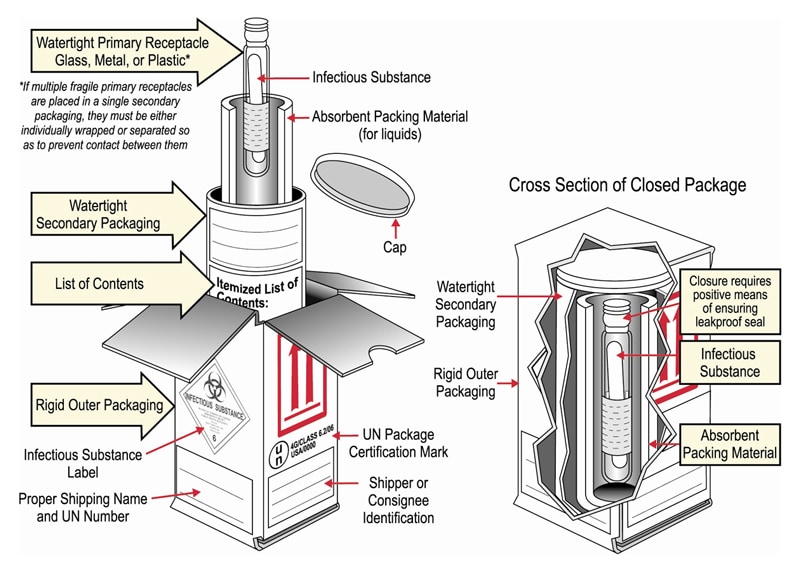
Frequently Shipped Biological Material and Proper Classification Professional judgment must be used; if you suspect the specimen may contain an infectious substance, it must be shipped accordingly. These shipments should be packaged using a triple packaging system and marked as "exempt human specimen" or "exempt animal specimen." Exempt patient specimens include: Biopsies. Dried blood spots.
PDF EXEMPT RESEARCH - University of California, Berkeley "Exempt" research are human subjects studies that present no greater than minimal risk to subjects and fit into one or more exempt categories (as described below).
Job Status: Exempt vs. Non-Exempt | Human Resources | Georgia Institute ... When calculating overtime, it is important to note that overtime is based on the number of hours worked in the workweek, not the pay period. Hours that are not actually worked (holidays, sick, vacation, etc.) do not count towards calculating overtime. Overtime must be approved in advance by the supervisor. The supervisor may also adjust the ...
What is "Exempt" Human Subject Research, And What Does It Mean? (2019 ... A study consisting of analyzing data or specimens only, and not involving any interventions or interactions with subjects to collect those data or specimens, is most likely to be exempt if it is not externally funded. Of the three categories listed below, only the first is available for externally-funded research, and it is quite restrictive.
Boxes - Sarstedt What do P650 "light" and "exempt human specimen" mean? This is an exemption under category B (infectious substances allocated UN3373), but only applies if there is a professional assessment (e.g. from a doctor) stating that the patient samples to be shipped have no or minimal likelihood of containing a pathogen.
Waived Tests | CDC Waived tests include test systems cleared by the FDA for home use and those tests approved for waiver under the CLIA criteria. Although CLIA requires that waived tests must be simple and have a low risk for erroneous results, this does not mean that waived tests are completely error-proof. Errors can occur anywhere in the testing process ...
PDF QUICK GUIDE - Using Human Biological Specimens at UC Davis identifiers linked to the specimens. If the investigator does not record the identifiers or link them to the specimens, the research may be eligible for review under the exempt category. Specifically, exempt category #4 applies to research that involves the collection or study of existing* data,













Post a Comment for "39 what does exempt human specimen mean"