42 multi step reaction energy diagram
ENERGY PROFILES FOR SIMPLE REACTIONS - chemguide Diagrams like this are described as energy profiles. In the diagram above, you can clearly see that you need an input of energy to get the reaction going. Once the activation energy barrier has been passed, you can also see that you get even more energy released, and so the reaction is overall exothermic. If you had an endothermic reaction, a ... CH 368: Unit 2 - University of Texas at Austin Conventionally, they are a plot of energy (in the rigorous case, free energy, G) versus the reaction coordinate. 6. Reaction Mechanism. The mechanism of a reaction is a stepwise description of how reactants are converted to products. Of course, some reactions occur in a single step, as represented in the reaction path diagram above. Such ...
PDF Energy Profile Diagrams - staff.du.edu.eg Energy Diagram for the Chlorination of Methane Chapter 4 5 Rate-Limiting Step • Reaction intermediates(e.g.CH 3 •) are reactive species however, they can be stable(i.e. less reactive) as long as they don't collide with another molecule or atom. • Transition statesare at energy maximums. • Intermediatesare at energy minimums.

Multi step reaction energy diagram
Energy Diagram for a Two-Step Reaction Mechanism Step 1 has the higher transition energy state, thus it is the rate-determining step. In many reactions more than one step is involved in the formation of products. Step Two Exothermic because energy is released in forming B-C bond delta H is negative Products are lower than the starting materials Solved 3. The energy diagram for a multi-step reaction | Chegg.com The energy diagram for a multi-step reaction mechanism for the decomposition of C4H9Br is shown below. Multistep Reactions - Softschools.com The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product.
Multi step reaction energy diagram. Chapter 6: Understanding Organic Reactions Flashcards - Quizlet A) The product is favored in reaction in which DH° is a positive value. B) Entropy decreases when an acyclic compound forms a ring. C) In homolytic bond cleavage, entropy decreases and favors formation of products. D) The starting material is favored in a reaction in which DH° is a negative value. B. Energy Diagrams of Reactions | Fiveable To find the activation energy, you should be looking for two numbers: the potential energy of the reactants and the energy of the activated complex (the maximum point). (energy of activation complex) - (PEreactants) (100 kJ) - (40 kJ) = 60 kJ In other words, it takes 60 kJ of energy to complete the reaction. Energy Diagram Module Series- Part Three: Intermediates and Rate ... Sometimes reactions are more complex than simply a transition state (Graph 3), which would represent a single step in the reaction mechanism. You will soon see most reactions proceed in a multistep fashion. In this case, reaction mechanisms often form lower energy and sometimes isolatable intermediates. How do you find the rate determining step from a graph? | Socratic Explanation: The rate determining step in a reaction mechanism is the slowest step. It is characterized by its high activation energy. Consider the energy diagram represented below of a two-step mechanism. The first step is the slow step since it has the highest activation energy. Here is more about this topic in the following video: Chemical ...
Chem 51A. Lecture 19. Organic Chemistry: Ch. 6. Energy Diagrams ... -11:11 Energy Diagram for Single Step Reaction-17:13 Transition State-26:06 Energy Diagram for Multi-Step Reaction-34:46 Steps in the Multi-Step Reaction-30:01 Reverse Reaction of Multi-Step Reaction-41:34 Rates of Reaction-47:15 Other Factors Affecting Rate. Required attribution ... Answered: Sketch an energy diagram for a two-step… | bartleby Con-sider this one-step reaction for the formation of this compound:NO (g)+Cl₂ (g) →NOCl (g)u0007+Cl (g) ΔH°u0006=83 kJ (a) Draw a reaction energy diagram, given Ea (fwd)is 86 kJ/mol. (b) Calculate Ea (rev). (c) Sketch a possible transition state for the reaction. arrow_forward 10. Reaction mechanism and rate law (article) | Khan Academy A reaction that occurs in two or more elementary steps is called a multistep or complex reaction. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. The slowest step in a reaction mechanism is known as the rate-determining step. Energetics: 4.32 - Hess' law energy cycles and diagrams An energy cycle is a diagram showing how three, or more reactions are interconvertable. Energy cycles can be constructed to find unknown energy changes, by adding up the energies of steps from a different route to the same final products. For example, if the energy of the conversion A B is known, and C can also be converted into B, C B, and ...
Multistep reaction energy profiles (video) - Khan Academy Next, let's look at the energy profile for this multi-step reaction. Energy profiles usually have potential energy in the y-axis and then reaction progress on the x-axis. So as we move to the right on the x-axis, the reaction is occurring. This first line on our energy profile represents the energy level of our reactants, which are BC and D. Reaction Mechanisms - Chem1 2 Multi-step (consecutive) reactions. Mechanisms in which one elementary step is followed by another are very common. step 1: A + B → Q. step 2: B + Q → C. net reaction: A + 2B → C. (As must always be the case, the net reaction is just the sum of its elementary steps.) In this example, the species Q is an intermediate, usually an unstable ... Energy Diagrams of Two Step Reactions - YouTube Watch Complete videos @ Organic Chemistry 1 7.4 SN1 Reaction Mechanism, Energy Diagram and Stereochemistry Because S N 1 is a multiple-step reaction, so the diagram has multiple curves, with each step can be represented by one curve. Out of the three steps, the activation energy for step 1 is the highest, therefore step 1 is the slowest step, that is the rate-determining step. Figure 7.4a Energy diagram for SN1 reaction between (CH3)3CBr and H2O
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
How to Draw Multi-Steps Energy Profile Diagrams: Reactant, Product, ∆H ... We'll go over how to label the reactants, products, transition state, activated complex, activation energy, ∆H delta H, forward reaction, reverse reaction. We'll go over how the activation energy...
Multi-Step Reaction - an overview | ScienceDirect Topics The multi-step reaction catalyzed by the PDH complex involves sequential reactions with the intermediates being relayed between the subunits via the flexible lipoyl domains. E1 catalyzes the decarboxylation of pyruvate forming 2- (1-hydroxyethyldiene)-TPP bound to E1 (TPP is thiamin pyrophosphate).
From the given energy diagram - step of the mechanism, ΔG , Ea and Keq ... Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram. ΔG = Gibbs free energy, ΔG (Gibbs free energy) of the reaction is mainly depends on the free energy of reactant and product.
Label the following multi-step reaction energy diagram. Label numbers 1-4 on the energy diagram: Label the activation energies on the diagram. Is the overall reaction endothermic or exothermic? 3. What is the reactant for the rate determining step? Reaction Path 27. Calculate the pH of a 0.0sS M solution of CHCNa (K,(C)-18x10)
Rate Determining Step for Reaction Coordinate Diagram Take two-step exothermic reaction where the FIRST transition state is HIGHER than the second transition state: The step with the higher activation energy, is the slow step (The first transition state). Because it has the higher activation energy, it will have the lower rate constant. The rate determining step would be the first step as well.
Label the following multi-step reaction energy diagram. - Chegg Solved Label the following multi-step reaction energy | Chegg.com. Science. Chemistry. Chemistry questions and answers. Label the following multi-step reaction energy diagram.
Potential Energy Diagrams - Kentchemistry.com A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values.
TIGER - NCSSM Distance Education and Extended Programs This diagram shows a potential energy diagram for a multi-step mechanism. NOplusH2_ver_1-640.gif This diagram shows a one-step mechanism for a reaction. NOplusH2_ver_2-640.gif This diagram shows a two-step mechanism for a reaction with the first step being rate determining. NOplusH2_ver_3-640.gif This diagram shows a two-step mechanism for a ...




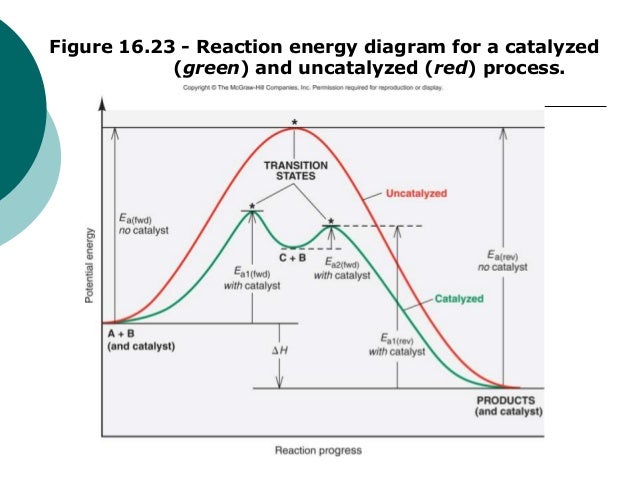
Post a Comment for "42 multi step reaction energy diagram"